In chemistry, we are starting a unit on organic chemistry– the study of carbon-based molecules. Organic molecules are ones with a carbon and hydrogen “backbone”. A special class of these molecules are hydrocarbons– these are structures comprised only of carbon and hydrogen.
Organic molecules are very abundant in our society- millions of them exist (compared to approximately 250,000 inorganic molecules). Until 1828, all organic molecules came from living or formerly-living organisms. In 1828, Friedrich Wohler synthesized urea from ammonium cyanate; this revolutionalized the way chemists looked and organic and inorganic molecules and opened the door to the creation of many synthetics.
The abundance of organic compounds can be attibuted to the properties of the carbon atom. Carbon is a versatile element for three reasons:
- Carbon atoms are able to form 4 covalent bonds. It can bond with up to 4 other atoms and it can bond to itself.
- Carbon can form single, double or triple bonds with itself. This allows carbon to form long chains that are stable, a rare attribute.
- Carbon can form many geometric structures. Some of these include chains, branched chains, sheets, rings, tubes and spheres. This is a unique characteristic.
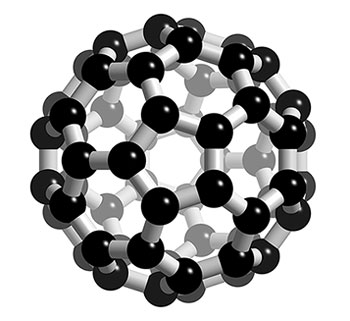
This is a Buckminsterfullerene, a sphere made up entirely of carbon (60 carbons are present). Image was borrowed from: http://www.3dchem.com/imagesofmolecules/c60.jpg
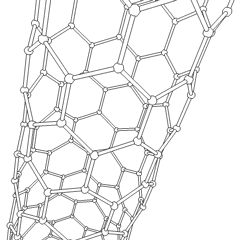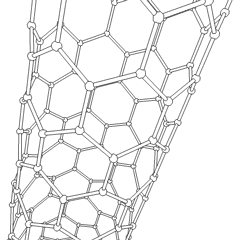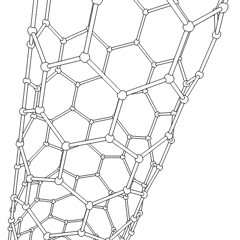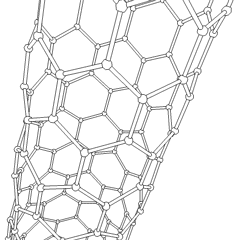
This is a depicition of a carbon-based nanotube. The structure has unique properties such as high tensile strength, high electrical conductivity, high ductility, high resistance to heat, and relative chemical inactivity. These are the types of structures that we saw at Composites Atlantic. Image was borrowed from: http://en.wikipedia.org/wiki/Image:Kohlenstoffnanoroehre_Animation.gif
Hydrocarbons can be represented in a number of ways:
- Complete structural diagram: all the atoms and the arrangement of bonds are shown.
- Condensed structural diagram: simplified, the bonds between the carbon atoms are shown but not between the hydrogens and carbons.
- Line diagram: lines are used to illustrate hydrocarbons. The end of each line and the points where lines meet indicate that a carbon is present. It is assumed that the other bonds are formed with hydrogen.
- Expanded molecular formula: this representation does not take much room but shows the grouping of molecules. Example: CH2CH(CH3)CH2CH2CH3

An example of a complete structural diagram. Borrowed from: http://www.ratical.org/renewables/hs2.gif
As we get into more complex molecules, we will begin to see that many hydrocarbons have isomers. Isomers are compounds with the same molecular formula but a different structural arrangement.
Homework: Please complete Investigation 9-A on page 328 of the text. You are expected to draw three diagrams of each of the 3 isomers for the first molecule (C5H12) but you only need to represent the second molecule (C7H16) with one type of diagram. Make sure you complete questions 1-5 (skip question 1if you did not make predictions). This will be reviewed and submitted on Thursday.
