Please find the rubric for the Environment and Energy paper attached below. Please be reminded that the paper is due by e-mail by Friday, March 27, 2009 by the the beginning of class. I strongly advise you to send your paper the night before and verify with me during colours whether or not I received it. If the paper is not in by the start of the class, you will receive a mark of zero. Please remember Mr. Cleland’s advice about ensuring that your paper is in the correct format.
Category Archives: Grade 11 Chemistry
Chemistry- Rubric for Environment and Energy Paper
Filed under Grade 11 Chemistry, Grade 12 Chemistry
Chemistry- Tuesday, Feb. 10, 2009
So far, we have introduced the law of conservation of energy and how it applies to different systems.
We completed the heat transfer activity which allowed us to test for the different factors that need to be considered when discussing heat transfer in a system.
You are expected to complete the handout for today’s activity and bring it to class tomorrow.
Filed under Grade 11 Chemistry
Exam Reminders- January 23, 2009
Just a few reminders for the upcoming exams….
- The only required material for the math, physics and chemistry exams are pencils, erasers and calculators. Other material (paper, periodic table, etc) will be provided.
- Please remember to bring all texts and other borrowed material to the exam. These text books will be collected at that time. You are accountable for your texts!
- Physics students are permitted to bring their formula sheet if there no notes written on them.
- Anyone with questions about the material can contact me via e-mail or see me during the time frame between exams.
Best of luck!
Chemistry- Electric Car Comes to Canada?
I recently found an interesting article on the potential development of an electric car infrastructure in Canada. I highly recommend reading this article- perhaps there is hope!
Filed under Grade 11 Chemistry
Chemistry- Oral Exam Schedule, January 16, 2009
Oral exams for chemistry will be taking place on Wednesday and Thursday of next week (in class). The schedule for exams:
Wednesday
- Rob
- Jackie
- Callagy
- Antoine
- Kate
Thursday
- Pat
- Jenn
- Mitchel
- Lily
- Daniela
- Sam
Chemistry- Information for the Final, January 15, 2009
Chemistry 11: Exam Information
General Exam Info
The grade 11 chemistry final is divided into 2 sections:
- Oral Component (10% of final grade)
- Written Component (40% of final grade)
The oral exam will be conducted on Wednesday January 21 and Thursday January 22 in class. The oral exam is 5-10 minutes long and will require you to discuss a topic that we studied in class.
The written exam will be on Tuesday January 27th from 9AM-12PM.
Review Dates
In-class review time will be provided on January 16, 19, 20 and the 21st or 22nd (differs for each student depending on the date of their oral exam).
Availability
There will be additional time available outside of class on the following dates:
Monday, January 19- 3:00-4:30PM (general)
Wednesday, January 21- 12-12:30PM (general), 3:00-4:00PM (chemistry only)
Thursday, January 22- 12-12:30PM (chemistry only)
Please bring questions or issues to tutorials to help structure the tutorial.
Topics Covered
All the topics covered in the semester may appear on the exam. There is greater weight on post-midterm material than there is on pre-midterm material.
- Avogadro’s Number
- Moles and Mole Conversion (how and when to use it)
- Molar Volume of Gases (how to apply it to problems)
- Percentage Composition of Compounds (how to find it and use it)
- Law of Definite Proportions
- Law of Combining Volumes
- Empirical Formulae (what is it, how to find it)
- Molecular Formulae (what is it, how to derive it)
- Hydrated Ionic Compounds (what are they, why are they significant, calculations with them)
- Balancing Equations (how to do it as well as how to use them in solving problems)
- Stoichiometry and Stoichiometric Calculations (importance and how to use it)
- Limiting Reactant (why it is used, solving for it, applying it to reactions)
- Percentage Yield (how to find it, why it is needed)
- Percentage Purity (how to calculate it and use it to determine product formation)
- Classifying Hydrocarbons (intro to organics, naming alkanes, alkenes, alkynes; combustion)
- Bond Formation (ionic, covalent, Lewis dot diagrams)
- Electronegativity
- Intermolecular Forces and Intramolecular Forces
- Symmetrical/Asymmetrical Addition to Alkenes, Cyclic Hydrocarbons, Aromatic Hydrocarbons
- Polymers
Physics- Review of Some Topics, Dec. 1, 2008
Just a quick review of some of the topics we have covered since the midterm…
Work
W= Fd
– work is defined as a transfer of mechanical energy
– it causes an object to change or move
– work is always done on an object
– is measured in N*m
– review the conditions for when work is done (work is not done hen there is no change in condition, there is uniform motion or if the force applied is perpendicular to the direction of the motion)
– Review practice problems #1-3 on page 221 and #4-10 on page 225 for a refresher
Momentum
p = mv
– the momentum an object possesses is related to the product of its mass by its velocity
– the direction of the momentum is the same as its velocity (is a vector)
– has a unit of kg*m/s
– used when dealing with non-elastic collisions- it’s easier to describe the “quantity of motion” of an object before and after a crash than to try and analyze it during the moment of
Impulse
J = F(delta)t
F(delta)t= (delta)p
F(delta)t = mv2-mv1
– is the product of the force exerted on an object and the time interval over which the force acts
– impulse is a measure of the change in momentm
– direction of impulse is the same as the direction of the F applied
– unit is in N*s
– Review problems 29 on 197, 30-32 on 200 and 33-35 on 203
For additional problems, check out: #37-42 on page 209 and #15, 17, 20, 23-28 on page 275 and 276.
Filed under Grade 11 Chemistry
Chemistry- Introduction to Organic Chemistry, November 25 and 26, 2008
In chemistry, we are starting a unit on organic chemistry– the study of carbon-based molecules. Organic molecules are ones with a carbon and hydrogen “backbone”. A special class of these molecules are hydrocarbons– these are structures comprised only of carbon and hydrogen.
Organic molecules are very abundant in our society- millions of them exist (compared to approximately 250,000 inorganic molecules). Until 1828, all organic molecules came from living or formerly-living organisms. In 1828, Friedrich Wohler synthesized urea from ammonium cyanate; this revolutionalized the way chemists looked and organic and inorganic molecules and opened the door to the creation of many synthetics.
The abundance of organic compounds can be attibuted to the properties of the carbon atom. Carbon is a versatile element for three reasons:
- Carbon atoms are able to form 4 covalent bonds. It can bond with up to 4 other atoms and it can bond to itself.
- Carbon can form single, double or triple bonds with itself. This allows carbon to form long chains that are stable, a rare attribute.
- Carbon can form many geometric structures. Some of these include chains, branched chains, sheets, rings, tubes and spheres. This is a unique characteristic.
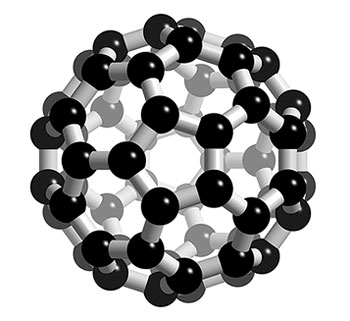
This is a Buckminsterfullerene, a sphere made up entirely of carbon (60 carbons are present). Image was borrowed from: http://www.3dchem.com/imagesofmolecules/c60.jpg
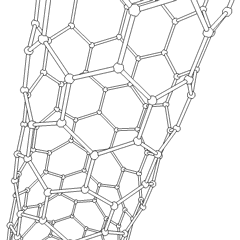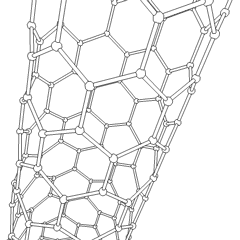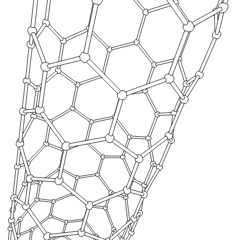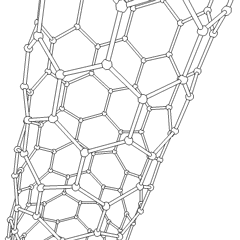
This is a depicition of a carbon-based nanotube. The structure has unique properties such as high tensile strength, high electrical conductivity, high ductility, high resistance to heat, and relative chemical inactivity. These are the types of structures that we saw at Composites Atlantic. Image was borrowed from: http://en.wikipedia.org/wiki/Image:Kohlenstoffnanoroehre_Animation.gif
Hydrocarbons can be represented in a number of ways:
- Complete structural diagram: all the atoms and the arrangement of bonds are shown.
- Condensed structural diagram: simplified, the bonds between the carbon atoms are shown but not between the hydrogens and carbons.
- Line diagram: lines are used to illustrate hydrocarbons. The end of each line and the points where lines meet indicate that a carbon is present. It is assumed that the other bonds are formed with hydrogen.
- Expanded molecular formula: this representation does not take much room but shows the grouping of molecules. Example: CH2CH(CH3)CH2CH2CH3

An example of a complete structural diagram. Borrowed from: http://www.ratical.org/renewables/hs2.gif
As we get into more complex molecules, we will begin to see that many hydrocarbons have isomers. Isomers are compounds with the same molecular formula but a different structural arrangement.
Homework: Please complete Investigation 9-A on page 328 of the text. You are expected to draw three diagrams of each of the 3 isomers for the first molecule (C5H12) but you only need to represent the second molecule (C7H16) with one type of diagram. Make sure you complete questions 1-5 (skip question 1if you did not make predictions). This will be reviewed and submitted on Thursday.
Filed under Grade 11 Chemistry
Chemistry- Hydrogen Bonding, November 20, 2008
Explaining Physical Properties with Bonding
Use what you know about intermolecular forces to explain the following situations.
1. a) How does the density of metal compare to the density of water?
b) If you fill a shallow Petri dish or cup with water and place a paper clip on top,
the paper clip will float. Why? Try to explain using what you know about bonds.
2. Research the boiling point of water. How does it compare to other liquids (example: H2S)? Why is it so much higher or lower?
3. a) Why does ice float on water? How do hydrogen bonds play a role?
b) In ecological terms, what is the impact of this phenomenon?
4. Water is often regarded as the universal solvent. Why is water so good at dissolving so many substances?
5. What is the role of hydrogen bonding in proteins? How does hydrogen bonding affect the shape?
6. Cut a strip of plastic and rub it with something wooly. Turn on a tap so there is a small, steady flow of water. What happens when you bring the strip close to the stream? Why does this happen? What do you suppose would happen if you did the same thing but with a stream of vegetable oil (non-polar substance)?
Bonus: What is the role of hydrogen bonding in plant life? Consider how plants “absorb” water through the roots. Can you explain this?
Please note that this assignment is due on Monday, November 24, 2008. You are encouraged to share your assignment through google docs (by 8AM on the morning of November 24) or if you wrote your answers, it is due at the beginning of class.
Filed under Grade 11 Chemistry
Ethics in Chemistry Overview
Ethics in Chemistry Assignment
You and your group will be researching different topics related to chemistry and then bringing your topic to class. The aim of this project is not to repeat information that you find on the internet or in other sources but to take that information and critically analyze all the aspects related to it. Your role as a group will be facilitators of a discussion in class about your topic. This project is meant to give you a chance to research and talk about different issues and discover different opinions.
As a group, you should be able to provide some background information on your topic. What is the historical relevance of your topic? What is the relevance to society? Where is this important? This section will provide the basis upon which people will form their opinions. This section should be presented in a neutral fashion.
The second segment is meant to be a chance for you to bring up some issues related to your topic and to come up with some discussion points for the class to ponder over. All the topics selected have controversial elements to them- it is important to critically analyze these elements and to consider the ethics of science.
Some questions to consider include:
– Was anyone affected by this science?
– Did anyone profit from this science?
– How was or is the environment treated? What are the effects and are they justified?
– Why might someone disagree with the use of the science?
– Does the pros of the science outweigh the cons?
– Is there someone who should be held accountable?
o Are they being held accountable?
o If no, why? What is protecting them?
– Is there a bias in the sources that you have found?
– What are your opinions on this issue?
– What are some solutions? Is there a solution?
The number of questions you can ask is infinite so please only use these to get you started. These are issues to consider incorporating into your discussion points. If you have issues with structuring your questions or discussion points, please see me and we can talk about them.
Role of a Facilitator
• Maintain a neutral role — prompt others to discuss their ideas and opinions.
• Maintain a balanced flow of ideas among the participants— don’t let a few individuals dominate the discussion.
• Ensure that everyone participates.
For individual topics, some specific considerations include:
DDT: How long have the effects been felt? Why would the use of DDT be justified? Unjustified? Are articles about Rachel Carson being the world’s worst serial killer valid? Are there parts that are valid to these arguments? What are the ecological effects? What are the rules regarding DDT right now?
Medicinal Patents: what is the purpose of a medicinal patent? How long do they last for? Who holds them? What are the ripple effects of patents? What are pros of patents and cons of patents? Are there any drugs that are made inaccessible due to patents? Is it fair to mandate the use of certain, patented drugs? What is the ethical responsibility of drug developers?
Nuclear Energy: Why is nuclear energy used? What are the benefits? Why do some groups argue so vehemently against them? Are their arguments fair or just emotional? Does nuclear energy have a place in society? What are the ecological effects?
Agent Orange: How long have the effects been felt? Was the use of DDT justified during the Vietnam War? What are the arguments for? What has happened to the people of Vietnam? Is any held accountable? What can be done now?
Ethics of Chemistry Rubric
Filed under Grade 11 Chemistry

