Thermochemistry
- Pg. 634, #1-4
- Pg. 637, #11-14
- Pg. 638, #1, 5, 6, 7
- Pg. 645, #19-23
- Pg. 685, Inquiry problems
- Pg. 676, #1, 2, 3, 7
- Pg. 681, #11-14
- Pg. 685, #15-18
- Pg. 687, #19-22
- Pg. 690, #23-26
- Pg. 691, # 1, 3, 4, 7
Solutions
- Pg. 254, #1-8
- Pg. 258-268, Odd #
- Pg. 261 #5-9
- Pg. 265 #15-18
- Pg. 268 #19-24
- Pg. 276 #1-5
Solution Stoichiometry
- Pg. 287 # 1,2, 3, 4
- Pg. 294 #5, 6
- Pg. 298 #2, 3, 5
- Pg. 300 #7-10
- Pg. 302 #11-14
- Pg. 304 #15-18
- Pg. 30619-21
Rates of Reaction (Kinetics)
- Pg. 467 #2
- Pg. 476 #1-4
- Pg. 484 #3, 4, 5, 9, 10, 11
|
Equilibrium
- Pg. 497#1-5
- Pg. 499 #6-10
- Pg. 508 #11-15
- Pg. 511 #16-19
- Pg. 515 #25-29
- Pg. 516 #1, 2, 3
- Pg. 529 #33-37
- Pg. 533 #1-3
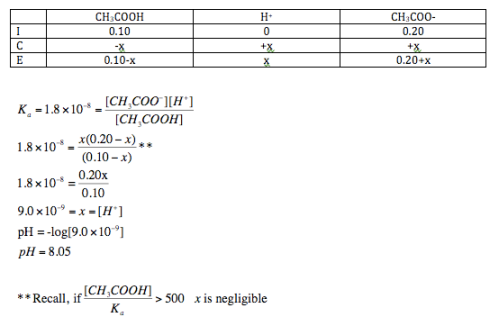
Acids and Bases
- Pg. 557 #1, 2, 6
- Pg. 564 #10, 11
- Pg. 566 #12-15
- Pg. 569 #16-19
- Pg. 572 #20-25
- Pg. 574 #26-29
- Pg. 586 #1-4
- Pg. 591 #5-10
- Pg. 595 #11-16
- Pg. 602 #17-20
Redox Reactions
- Pg. 716 #5-8
- Pg. 726 #9-12
- Pg. 728 #13-16, #2
- Pg. 732 #17-20
- Pg. 734 #21-24
- Pg. 738 #25-28
|